Diagnostic testing of the ocular surface and anterior segment remains a vital part of the ocular examination for determination of vision-threatening diseases. Amazing advancements in new technology have simplified disease identification and enabled clinicians to make appropriate decisions about disease-altering treatments for the anterior segment. Herein we review some of the groundbreaking diagnostic equipment now available to ophthalmologists in the United States.
Anterior Segment OCT
Anterior segment optical coherence tomography (AS-OCT) uses light waves to create high-resolution, cross-sectional images of anterior segment structures, including the cornea, anterior chamber, iris, angle, and crystalline lens. This technology provides diagnostic advantages in assessing the depth and extent of corneal pathology, ocular surface tumors, and cysts; identifying and following patients with keratoconus; and planning for refractive and post-refractive cataract surgery. With the wide adoption of AS-OCT in the field of ophthalmology, clinicians can see structures of the eye that previously were not visible.
KEY TAKEAWAYS:
- High-tech imaging enhances eye disease diagnosis
- Anterior segment OCT offers detailed structural insight
- Corneal tomography detects early keratoconus
- Confocal microscopy aids in diagnosing infections
- Imaging supports precision treatment decisions
High-resolution AS-OCT can be particularly helpful in diagnosing ocular surface lesions. The device can delineate between intraepithelial and invasive ocular surface tumors.1 Characteristics of intraepithelial ocular surface squamous neoplasia (OSSN) include a clear plane of separation between thick hyperreflective epithelium and hyporeflective areas and a hyperreflective basal membrane.1 Moreover, hyporeflective zones within the tumor that are surrounded by thickened hyperreflective epithelium were found to be characteristics of invasive OSSN.1 For melanocytic tumors, a thick basal epithelial hyperreflective band with normal overlying epithelium without cysts has been shown to be a characteristic of primary acquired melanosis (PAM).2 Conjunctival melanomas typically have epithelium of normal thickness with a variable hyperreflectivity of the basal layer and the lesion itself being subepithelial.3 Anterior segment OCT images of ocular surface lesions can aid in the determining of whether to biopsy a suspicious conjunctival lesion vs simply observing it over time.
Additionally, high-resolution OCT allows for epithelial mapping. This is a newer feature that can be particularly useful in diagnosing keratoconus vs other pathologies—for example, contact lens warpage. Epithelial mapping can also aid in identifying patients with keratoconus progression, and can be useful in determining which patients may benefit from collagen crosslinking. Post-crosslinking, it can track patients’ improvement.
OCT imaging may also be helpful in choosing treatment options for corneal disease by determining the depth of a corneal scar or dystrophic lesions. If the pathology is superficial, a phototherapeutic keratectomy (PTK) can be used to remove the pathology and improve cornea clarity. If OCT shows that pathology extends to deeper layers of the cornea, a deep lamellar keratoplasty may be warranted. If pathology appears to be full thickness, a penetrating keratoplasty may be the best option for improving cornea clarity. The OCT can also show depth of keratolysis in infectious keratitis cases.
Corneal Tomography
Corneal tomography is an advanced diagnostic tool that uses Scheimpflug imaging to take photographs of the anterior and posterior cornea, the latter of which can be abnormal in early onset keratoconus. There are many types of corneal tomographers on the market; 2 of the most common machines available in the US are the Pentacam (Oculus Optikgeraete GmbH) and the Galilei G4 or G6 (Ziemer Ophthalmic Systems AG). The devices can simultaneously capture placido disk images of the anterior cornea along with Scheimpflug images. They also have numerous metrics to screen for keratoconus. Depending on the device purchased, parameters may include the keratoconus probability index (KPI), inferior-superior index (I-S), irregular astigmatism index (IAI), and differential sector index (DSI) (Figure 1).
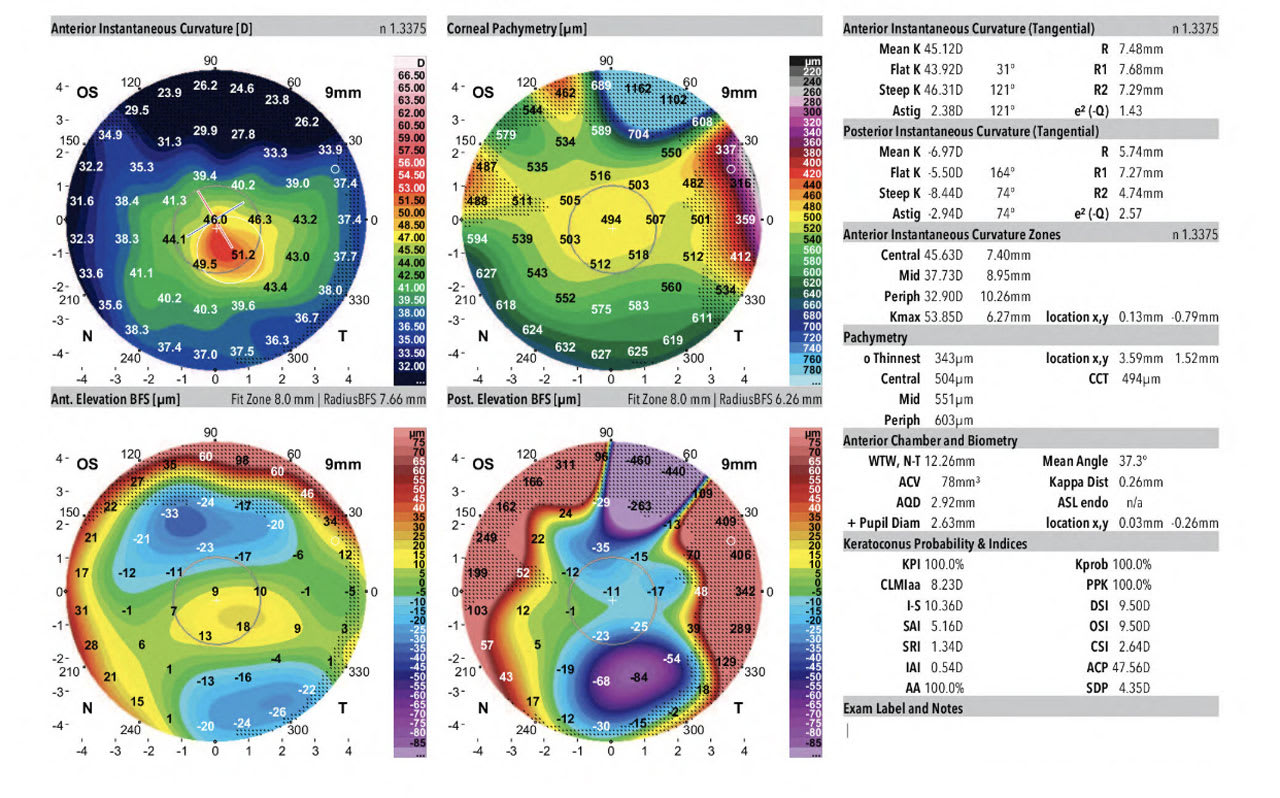
The Belin/Ambrósio Enhanced Ectasia Display (Release II) of the Pentacam simplifies the physician’s evaluation of refractive surgery patients, particularly helping to identify patients at risk of or showing signs of keratoconus. The new display reports 5 differential parameters individually, including a change in anterior elevation from standard to enhanced reference surface; change in posterior elevation; corneal thickness at the thinnest pachymetry; thinnest pachymetry displacement; and pachymetric progression. A value of less than 1.6 SD is considered low risk for keratoconus, while a value greater than 2.6 SD is considered high risk.4
The Belin-Ambrosio display can be particularly useful in determining the presence of keratoconus when deciding whether refractive surgery is safe and at low risk of creating cornea ectasia. Cutoff thresholds for the diagnosis of clinical keratoconus and pre-keratoconus have been studied in each of these metrics.4 On the Galilei imaging device, KPI values of 10% or less; I-S ratio of 1.6 or less; IAI of 0.45 or less; and DSI of 1.72 or less are generally accepted as cutoff values for pre-keratoconus.5 The higher each value, and the more values that are elevated simultaneously, give the physician a higher index of suspicion that keratoconus or corneal ectasia is present. Therefore, clinicians can say with a large degree of certainty that a patient has pre-keratoconus or clinical keratoconus.
Confocal Microscopy
One of the most common conditions that we see as cornea specialists is infectious keratitis, with contact lens wear as the most common risk factor. Most of these patients have mild disease that is bacterial in nature and due to trauma, improper contact lens wear, or poor hygiene. However, severe cases can occur from a variety of pathogens, and the clinical exam does not always reveal which organism is responsible. Atypical pathogens can be challenging to recover on cultures and may be misdiagnosed for bacterial or viral keratitis at the initial onset of infection.
Confocal microscopy is particularly helpful in diagnosing cases of fungal keratitis and Acanthamoeba keratitis. Characteristic findings in Acanthamoeba keratitis on confocal microscopy are the presence of hyperreflective cysts 15-28μm in size.6 They can be arranged in chains and clusters or in a single-file arrangement. Some research has found that the presence of cysts in chains and clusters portends a worse visual prognosis. Visualizing the cysts on confocal microscopy has a diagnostic sensitivity of 90% and specificity of 100%.6
For fungal keratitis, the hyphae of filamentous fungi such as Fusarium and Aspergillus species appear as numerous high-contrast lines 200-300μm in length that branch at an angle.7 For Candida species, these appear as many high-contrast elongated particles 10-40μm in size representing pseudohyphae.7 More-over, the presence of inflammatory cells is also visible in the epithelial layer in nearly all cases of fungal keratitis.
Confocal microscopy also can be very helpful in cases of infectious interface keratitis following endothelial keratoplasty. The classic interface fungal infiltrates in these cases are in the deep cornea and remain impossible to culture using traditional methods—thus, confocal scans can really aid in early diagnosis of these cases. Imaging is less helpful when significant corneal scarring or opacification is present. The view through the cornea must be somewhat transparent for the test to capture useful images. Confocal microscopy would not be helpful for a diffuse corneal infiltrate or an ulcer that completely obstructs the view into the anterior chamber.
Each of these anterior imaging technologies brings patients closer to effective treatment. OM
References
1. Singh S, Mittal R, Ghosh A, et al. High-Resolution Anterior Segment Optical Coherence Tomography in Intraepithelial Versus Invasive Ocular Surface Squamous Neoplasia. Cornea. 2018 Oct;37(10):1292-1298.
2. Alzahrani YA, Kumar S, Abdul Aziz H, et al. Primary acquired melanosis: clinical, histopathologic and optical coherence tomographic correlation. Ocul Oncol Pathol. 2016 Apr;2(3):123-7.
3. Shousha MA, Karp CL, Canto AP, et al. Diagnosis of ocular surface lesions using ultra-high-resolution optical coherence tomography. Ophthalmology. 2013;120(5):883-891.
4. Ambrosio R, Jr, Belin MW. Imaging of the cornea: topography vs tomography. J Refract Surg. 2010;26:847–9.
5. Feizi S, Yaseri M, Kheiri B. Predictive ability of Galilei to distinguish subclinical keratoconus and keratoconus from normal corneas. J Ophthalmic Vis Res. 2016;11(1):8–16.
6. Tu E, Joslin CE, Sugar J, et al. The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of acanthamoeba keratitis. Cornea. 2008;27:764–772.
7. Brasnu E, Bourcier T, Dupas B, et al. In vivo confocal microscopy in fungal keratitis. Br J Ophthalmol. 2007 May; 91(5): 588–591.









